The Role of Radiation Therapy in the Treatment of Malignant Uterine Tumors
Authors
INTRODUCTION
Radiation has a prominent role in the curative treatment of endometrial cancer and can be used before or after surgery or alone. High cure rates in adenocarcinoma of the endometrium have been reported in series combining surgery with some form of radiation. Radiation has also been used as an adjunct to surgery for uterine sarcomas. This chapter discusses the indications for radiation, techniques, and results.
RADIATION ALONE FOR MEDICALLY INOPERATIVE ADENOCARCINOMA
In early stage adenocarcinoma of the endometrium, surgery with or without radiation is the generally accepted mainstay of therapy. Unfortunately, many patients with endometrial cancer present with medical conditions in which surgery is contraindicated. In these patients, radiation becomes the only curative option. Brachytherapy alone or in combination with external-beam radiation therapy (EBRT) has been used. The overall 5-year survival rates for patients in whom radiation is used alone are 40–60%,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 whereas the survival rate for patients undergoing surgery with or without radiation is significantly higher.13, 14 Although direct comparison of survival is difficult because of intercurrent deaths in the radiation-alone group, pelvic failure rates also tend to be higher in patients treated with radiation alone.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Rose and associates13 used a case–control study to compare treatment results in patients who received primary radiation therapy versus surgery. They noted no statistical difference in survival.
Radiation can be delivered with a combination of EBRT and intracavitary irradiation or with intracavitary irradiation alone. Heyman and associates reported the use of intracavitary irradiation alone in 163 patients treated with three radium insertions using the packing method of Heyman.15 The 5-year overall survival rate was 46.6%. Kupelian and associates2 also reported a series of patients treated primarily with intracavitary irradiation. They noted a 14% 5-year uterine recurrence rate and an extrauterine pelvic recurrence rate of only 2%. Other series have also reported high local failure rates.3, 4, 5, 10 The series reported by Rouanet and colleagues3 noted a 24.2% 5-year local failure rate even though all patients received EBRT. Grigsby and coworkers6 noted a reduced pelvic failure with the addition of EBRT to intracavitary irradiation. In that group of 49 patients treated with both intracavitary and high-dose EBRT, the 5-year survival was 85.4%. These results were updated in 1995 to include a total of 101 patients. Overall 5- and 10-year survival rates were 66% and 38%, respectively. Disease-free survival rates at 5 and 10 years were 84% and 82%, respectively. Seventy-two of the 101 patients were treated with a combination of external beam and implant.7 Patanaphan and associates8 also noted an increased survival rate in patients who received combined EBRT and intracavitary irradiation (67%) compared with patients who received intracavitary irradiation alone (57%).
High-dose-rate (HDR) brachytherapy in medically inoperable patients has not been as widely studied as low-dose-rate (LDR) brachytherapy. A largest series was reported by Knocke and associates.12 In this study, 280 patients were analyzed, with the majority being clinically stage I and treated with HDR alone. Overall 5- and 10-year survival rates were 52.7% and 27.7%, respectively. Local control rates at 5 and 10 years were 75.4% and 70%, respectively. A report from Canada of 27 patients with clinical stage I and stage II disease noted a 15% pelvic failure rate and an 11% rate of late, serious complications.9 Nguyen and Petereit11 reported on 36 patients with clinical stage I disease treated with HDR alone. They noted an excellent uterine control rate of 88%, although this was associated with a significant complication rate. Modifications in technique have reduced the complication rate. Coon and colleagues reported 10-year result with using Rotte “Y” applicator for high dose rate brachytherapy in 49 patients with medically inoperable endometrial cancer. Five patients had acute grade 1 or 2 toxicity, and four patients had late grade 2 or 3 toxicity. The 3- and 5-year actuarial cause-specific survival rates were 93% and 87%, respectively. Overall survival rates were 83% and 42% at 3 and 5 years, respectively.16 In conclusion, primary radiation therapy in medically unresectable endometrial cancer produces good pelvic control and disease-specific survival. The treatment techniques vary, but intracavitary irradiation is the mainstay of treatment with some series advocating the addition of external beam radiation therapy for some or most of the patients.
PATTERNS OF RECURRENCE WITHOUT RADIATION THERAPY
When deciding on whether or when to use radiation therapy as an adjunct to hysterectomy, surgeons are required to have knowledge of the patterns of failure with surgery alone. Between 1977 and 1983, the Gynecologic Oncology Group (GOG) entered 1180 patients into a prospective study (Protocol No. 33) of early stage disease; the goal of the study was to relate surgical-pathologic parameters and postoperative treatment to recurrence-free interval and recurrence site. Table 1 relates recurrence to grade and depth of myometrial invasion in patients with no risk factors who were treated with surgery alone. Risk factors included positive nodes, adnexal spread, capillary space involvement, isthmus/cervix involvement, positive cytology, and gross disease outside the uterus. The site of recurrence is given when available. These data show that in patients with grade 1 or 2 disease and no myometrial involvement, the risk of recurrence with surgery alone is low and adjuvant radiation therapy probably is not indicated. However, despite negative risk factors, patients with high-grade or deep myometrial invasion are at significant risk for recurrence.14
Table 1. Gynecologic Oncology Group Protocol No. 33: Recurrence related to grade and myometrial invasion; surgery alone with negative risk factors
No Invasion | Inner Third | Middle Third | Outer Third | |
Grade 1 | 0/55 | 5/61 (8%) (2P, 1V) | 0/4 | |
Grade 2 | 0/17 | 2/41 (5%) | 1/7 (14%) (1V) | 1/2 (50%) (1V) |
Grade 3 | 1/5 (20%) | 2/7 (29%) (1V) | 1/1 (100%) (1P) |
P, pelvic failure; V, vaginal failure.
Similarly, Eifel and associates17 reported a recurrence rate of 0.8% (1/127) in patients with noninvasive tumors treated with surgery alone. This recurrence occurred in a patient with an initial grade 1 endometrial carcinoma in whom an anaplastic carcinoma of the pelvic side wall developed, which the authors believed to be a second primary; it was, however, scored as a recurrence.
Price and colleagues18 also studied the pattern of recurrence in patients with stage I disease treated with surgery alone. They noted a vaginal recurrence rate of 4.4%, 5.7%, and 13.6% for well, intermediate, and anaplastic histology, respectively. In the same group, the incidence of recurrence was 3.7% with no myometrial invasion, 4.7% with superficial invasion, and 15.1% with deep myometrial invasion.
Patients with pathologic stage II disease treated with hysterectomy alone are at a higher risk of recurrence than those whose disease is classified as pathologic stage I. The GOG study noted recurrence in seven of 29 patients (four pelvic, one vaginal) treated with surgery alone. Therefore, in this group of patients, the local recurrence rate was approximately 20% in those who did not receive radiation therapy.14
The current International Federation of Gynecology and Obstetrics (FIGO) staging system for stage II disease separates endocervical glandular involvement from stromal involvement.19 In a review by Fanning and coworkers,20 no patient with stage IIA disease treated with surgery alone had a recurrence compared to five of six patients with stage IIB disease. Other investigators have noted that in patients with stage II disease, histologic grade and depth of invasion remain important prognostic variables.21, 22, 23 Therefore, recurrence rates in patients with stage IIA disease probably are influenced greatly by other known prognostic variables.
Lymph-vascular space invasion has also been noted to be a risk factor for recurrence. Tsuruchi and associates24 noted a recurrence rate of 30.7% in clinical stage I and stage II patients with lymph-vascular space invasion versus 3.2% in patients who had no invasion. Similar increased recurrence rates have been noted by other authors.25, 26
Age is also a prognostic factor for survival. Younger women tend to have a better prognosis than older women. For instance, the GOG reported survival rates of 96.3% for patients ≤50 years old, 87.3% for patients 51–60 years old, 78% for patients 61–70 years old, 70.7% for patents 71–80 years old, and 53.6% for patients older than 80.27 As a general guideline, for every 1 year increase in age, the risk of recurrence increases by 7%.28
Patients with stage III disease represent a highly variable group. Patients with extrauterine spread limited to the peritoneal fluid or adnexa or both generally have more favorable outcomes compared to patients with other intra-abdominal metastases. In the GOG study of patients with stage IIIA disease who were treated with surgery alone, the recurrence rate was 0% (0/2) for adnexal involvement and 7% (1/14) for positive cytology. This compares with a recurrence rate of 50% in patients with positive pelvic nodes.14
Lymph node metastasis is the most important prognostic factor in clinical early-stage endometrial cancer. Of patients with cT1 disease, 10% will have pelvic and 6% will have para-aortic lymph node metastases.29 Patients with lymph node metastases have an almost 6 times higher risk of developing recurrent cancer than patients without lymph node metastases. One study showed a recurrence rate of 48% for patients with positive lymph nodes, including 45% with positive pelvic nodes and 64% with positive aortic nodes, compared to 8% for patients with negative nodes. The 5-year disease free survival rate for patients with lymph node metastases was 54% compared with 90% for patients without lymph node metastases.30
The recurrence rates for papillary serous histology, even when confined to the uterus, range from 50% to 85%, with upper abdominal recurrences predominating.17, 31, 32, 33, 34, 35, 36, 37 The histologic feature of papillary architecture alone does not appear to increase the recurrence rate,34, 35 although some authors have suggested that this presents some increased risk.38, 39 In patients with papillary serous histology, adjuvant radiation therapy would need to address the whole abdomen and is discussed later. Clear cell carcinoma has also been noted to have a higher recurrence rate.32, 40, 41
THE ROLE OF RADIATION IN OPERABLE STAGE I ENDOMETRIAL ADENOCARCINOMA
There have been numerous single-institution reviews and a few prospective, randomly assigned trials addressing the role of adjuvant irradiation. When combined with surgery, radiation can be given either before or after surgery. Advocates of preoperative irradiation state that the benefits include irradiating the tumor with an intact blood supply with a possible reduction in subsequent distant metastases and a questionable decreased risk of radiation side effects. Postoperative irradiation has the advantage of prior staging to help determine the need for irradiation and the areas at risk.
Aalders and coworkers42 published a trial of 540 clinical stage I patients randomly assigned to postoperative vaginal irradiation with or without additional EBRT. The patients who received additional EBRT had a pelvic/vaginal recurrence rate of 1.9% versus 6.9% in patients who were not given additional irradiation. No survival advantage was seen with EBRT. With additional evaluation, the authors concluded that patients with grade 3 disease who had more than half myometrial invasion benefited significantly from additional EBRT. The authors also recommended irradiation in cases of vascular invasion, given the poor prognosis shown in these lesions.
Piver and associates43 reported their results from a prospective, randomly assigned trial in clinical stage I patients comparing hysterectomy alone versus preoperative uterine irradiation versus postoperative vaginal irradiation. They noted more vaginal recurrences in patients who had received a hysterectomy alone (7.5%) than in patients treated before surgery (4.5%); none of the patients treated after surgery had a vaginal or pelvic recurrence.
In multiple, nonrandomly assigned reviews, authors have attempted to define the role of radiation in stage I disease. Piver and colleagues44 reported their results from a prospective trial using postoperative vaginal irradiation in patients with grade 1/2 disease who had invasion of less than 50% and no other evidence of disease. Patients with grade 3 disease or deep myometrial invasion received postoperative EBRT (group II). No patient in group I had a recurrence, and only one patient in group II had a pelvic recurrence. Grigsby and associates45 reported the results of a study of 858 clinical stage I patients, most of whom received preoperative intracavitary irradiation. Patients with deep myometrial invasion received EBRT. Only 1% of these patients had an isolated pelvic recurrence, and 3% had pelvic and distant recurrences. Nori and coworkers,46 using vaginal and selected EBRT either before or after surgery, noted a significant reduction in recurrences and improvements in survival compared with those of historical control subjects who had received surgery alone. Similar excellent pelvic and vaginal control rates have been noted in multiple series combining surgery and radiation.47, 48, 49, 50, 51, 52, 53
A survey of American gynecologic oncologists was undertaken to analyze surgical staging and its effect on adjuvant treatment recommendations in stage I endometrial carcinoma. For patients without lymph node metastasis, the majority of gynecologic oncologists recommended radiation for patients with grade 3 lesions or deep invasion or both. The recommendations for grade 1 and grade 2 lesions and lesions that are not deeply invasive were more variable.53
To define the role of radiation therapy in intermediate risk endometrial adenocarcinoma, the GOG performed a prospective, randomly assigned trial (GOG-99). All patients received complete surgical staging and were found to have stage IB, IC, IIA (occult), or IIB (occult) disease. The patients were randomly assigned to no additional therapy or 50.4 Gy of whole-pelvic radiation therapy. A total of 390 eligible patients were randomly assigned. The estimated 2-year, progression-free interval was 88% in the nontreated group versus 96% in the radiation therapy group (p = 0.004). There were 17 pelvic/vaginal recurrences in the nontreated group versus three in the radiation therapy group (two patients who refused radiation therapy). The estimated 3-year survival was 89% in the no-additional-therapy group versus 96% in the radiation therapy group (p = 0.09).54 The 5-year survival rates, 92% versus 86%, though not significant, favored the radiation group. An unplanned subset analysis was conducted in an attempt to define a group of patients with increased risk of recurrence. This group, based on prognostic factors including high grade, advanced age, deep myometrial invasion, or lymphovascular space involvement was defined as high–intermediate risk (HIR). The 2-year cumulative incidence of recurrence was 26% in the observation group versus 6% in the radiation group.55
Results of a randomly assigned study from the Netherlands (PORTEC trial) were reported by Creutzberg and associates.56 In this trial, patients were randomly assigned to pelvic radiation therapy (46 Gy at 2 Gy/fraction) versus no further therapy. Eligibility criteria included any adenocarcinoma including papillary-serous and clear cell, postoperative FIGO stage I, grade 1 with deep (greater than 50%) myometrial invasion, grade 2 with any invasion, and grade 3 with superficial (less than 50%) invasion. Peritoneal cytology was recommended but not required. In all 714 patients were entered and evaluable. The majority of patients were histologically adenocarcinoma. Approximately one-third of patients were FIGO stage IB, grade 2. There were six grade 3 complications and one grade 4 complication in the radiation therapy group versus one grade 3 complication in the surgery-alone patients. Five-year locoregional recurrences were noted in 14% of the untreated patients versus 4% in the radiation therapy patients (p <0.001). Overall 5-year survival was 85% in the control group versus 81% in the radiation therapy group (p = 0.37). Following subsequent central pathology review, there was a substantial shift from grade 1 to grade 2 lesions that would not have been eligible for inclusion in the study. Exclusion of these cases from analyses yielded essentially unchanged results, with 10-year recurrence rates of 5% for the radiation therapy group and 17% for the control group (p <0.001), and 10-year overall survival rates of 65% and 70%, respectively. Additionally a subset analysis was conducted on patients with at least two of three risk factors (grade 3 lesions, outer 50% myometrial invasion, and age ≥60 years) who were found to have increased risks of locoregional relapse. The 10-year rates of locoregional recurrence in this high-risk group were 4.6% in the radiation therapy group and 23.1% in the control group.57
These two randomly assigned studies, GOG-99 and the Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC) trial from the Netherlands, both seem to support the ability of radiation therapy to improve locoregional control in early stage endometrial cancer. This benefit is seen despite the inclusion of relatively lower risk patients with stage IB disease. The GOG trial also notes a strong trend to an improved survival.
The significantly improved locoregional control demonstrated by adjuvant radiation therapy in the PORTEC-1 trial was achieved primarily by a reduction in vaginal recurrence as compared to the control arm.14 Vaginal brachytherapy alone has been shown in many single-institution nonrandomized trials to have a low rate of recurrence in properly selected patients.58, 59, 60, 61, 62, 63, 64
The ASTEC and EN.5 trials were randomized trials in which 906 patients were randomized to adjuvant pelvic external beam radiation therapy (40–46 Gy in 20–25 fractions) or no adjuvant external beam radiation therapy. Thus far, the data have only been presented in oral presentation format at the ASCO 2007 annual meeting. Vaginal brachytherapy could be used regardless of the external beam randomization and was delivered as 4 Gy in two fractions (HDR) or 15 Gy via LDR. Treatment centers were required to decide in advance whether they would offer brachytherapy to all patients or to no patients. Brachytherapy was given to 52% of patients in each arm. Morbidity was 56% in the external beam radiation therapy arm compared to 24% in the no external beam radiation therapy arm. At a median follow up of 46 months, the 5-year hazard ratio for radiation therapy for overall survival was 1.01 (p = 0.98). The 5-year hazard ratio for radiation therapy for disease-specific survival was 1.17. The hazard ratio for an isolated pelvic or vaginal recurrence was 0.53 for the group receiving external beam radiation therapy. There is a small but significant decrease in pelvic recurrence with pelvic external beam radiation therapy.65
The PORTEC-2 was designed to compare postoperative EBRT to postoperative vaginal brachytherapy (VBT) in 427 patients with high-intermediate risk endometrial cancer. For this trial, high–intermediate risk was defined as (1) age ≥60 and stage IC grade 1–2, (2) age ≥60 and stage IB grade 3, or (3) any age and stage IIA grade 1–2, or grade 3 with <50% myometrial invasion. At a median follow up of 36 months, 3-year actuarial rates of vaginal relapse were 0.9% in the VBT arm and 1.9% in the EBRT arm (p = 0.97). Pelvic relapse rate was 6.3% with EBRT arm and 0.6% for the VBT arm (p = 0.03). Distant recurrence rate was 5.7% in the VBT arm and 6.3% in the EBRT arm. Three-year rates of vaginal, pelvic, and distant relapse as first failure were 0%, 1.3%, and 6.4% in the vaginal brachytherapy group and 1.6% , 0.7%, and 6.0% in the pelvic RT group. The 3-year overall survival rates were 90.3% for EBRT versus 90.8% for VBT (p = 0.96). The 3-year recurrence free survival was not significantly different. Gastrointestinal grade 1 toxicities were 35% in the EBRT arm versus 12% in the VBT group. Grade 2 toxicities were 19% in the whole pelvis radiation therapy group versus 7% in the VBT group (p ≤0.001). Grade 1 skin toxicities were 6% for the EBRT group and 1% for the VBT group, and grade 2 toxicities were 3% and 0%, respectively (p ≤0.001). The authors concluded that VBT should be the treatment of choice for patients with high intermediate risk of recurrence.66
STAGE II DISEASE
Treatment of stage II disease varies from radiation therapy alone to radical hysterectomy to a combination of surgery and radiation. Treatment of patients with stage II disease with radiation alone has generally resulted in much lower control and survival rates than when radiation and surgery have been combined.67 In addition, patients with cervical disease detected before surgery have been noted to have a worse prognosis than those patients with occult disease.67
Patients presenting with clinical stage II disease have commonly been treated with preoperative irradiation followed by extrafascial hysterectomy. The 5-year survival rates in patients who have received a combination of preoperative EBRT, intracavitary irradiation, and hysterectomy range from 69% to 88%.68, 69, 70, 71, 72, 73 The local control rates in these series are excellent. Grigsby and colleagues71 noted an 8.9% overall pelvic failure rate. Bruckman and associates69 noted no isolated pelvic failures and an overall pelvic failure rate of only 5%.
Radical hysterectomy alone has also been advocated as the treatment of choice by some authors. Boente and coworkers74 noted a lower recurrence rate and complication rate in patients undergoing radical hysterectomy compared with patients treated with radiation therapy and extrafascial hysterectomy. Arguments against radical hysterectomy have included the observation that many patients with endometrial cancer are elderly or obese and thus have significant comorbidities. In addition, if the decision to add EBRT is made after surgery, a higher complication rate can be expected. Given the high false-positive rates of endocervical curettage, radical hysterectomy should probably be considered only in cases that include gross cervical involvement.
A treatment approach that has gained favor in patients with stage II disease is initial extrafascial hysterectomy lymph node sampling and cytology followed by irradiation. This approach has resulted in patient survival rates comparable to those seen in patients who received preoperative irradiation and has also resulted in excellent pelvic control rates.23, 75, 76, 77
STAGE III DISEASE AND STAGE IVA DISEASE
Stage III or stage IVA disease can be separated into clinical and pathologic. Multiple series have noted an increased recurrence rate when irradiation alone is used.78, 79, 80 Patients with pathologic stage III disease have a better prognosis compared to patients with clinical stage III disease.81, 82 The role of radiation in stage III/IVA disease needs to be individualized for the extent of disease in each particular patient. In postoperative patients with positive pelvic lymph nodes, adnexal disease, serosal or parametrial spread, vaginal metastasis, or bladder/rectal invasion, pelvic irradiation with or without a vaginal-cuff boost should be considered. Using this algorithm, most series report 5-year survival rates of approximately 40–50% in patients with pathologic stage III disease.79, 80 Local control is accomplished in the majority of patients. In certain situations, there may be a role for extended-field and whole-abdominal irradiation.
Extended-field irradiation
The use of extended-field irradiation is limited to patients at high risk for extrapelvic recurrence. The clearest indication appears to be in patients who have evidence of para-aortic lymph node metastases as the only evidence of disease outside the pelvis. Extended-field irradiation refers to irradiating the pelvis, the common iliac, and the para-aortic lymph nodes.
Potish and associates83 reported their results in irradiating 40 women, all of whom had evidence of para-aortic lymph node metastasis. They reported a 47% 5-year survival in surgically staged patients, with only one severe complication. These results compare to a 10% 5-year survival in previous series that did not use extended-field irradiation.84 Rose and colleagues85 compared 17 patients who received extended-field irradiation to nine who did not. The survival in the extended-field irradiation group was 53% compared to 12% in the nonirradiated group, despite one treatment-related death in the former group. Other authors also noted relatively good survival in patients who received extended-field irradiation.86, 87
Whole-abdominal irradiation
The role of whole-abdominal irradiation in endometrial carcinoma remains controversial. Whole-abdominal irradiation has been used in a variety of patients ranging from those who received adjunctive therapy for high-risk surgical stage I disease88 to those with intraperitoneal metastatic disease.89 Whole-abdominal irradiation is used when there is a risk of intra-abdominal spread that may be impacted by treatment.
A number of authors have advocated the use of whole-abdominal irradiation in treating surgical stage III patients. Gibbons and coworkers88 noted a 57.8% 7-year disease-free survival in patients with surgical stage III disease who were treated with whole-abdominal irradiation. Potish and associates90 also noted an excellent 5-year relapse-free survival of 90% in patients with adnexal metastases or positive peritoneal cytology compared to zero in patients with macroscopic spread of cancer beyond the adnexa. The Gibbons article noted that three of a total of 27 patients treated with whole-abdominal irradiation had significant long-term bowel toxicity.88 The Potish article noted that only one of 27 patients had significant long-term bowel toxicity, although these investigators used a lower dose of whole-abdominal irradiation.89
Loeffler and colleagues91 reported the Joint Center experience with the use of whole-abdominal irradiation in 16 patients. They concluded that patients with extensive extrauterine involvement and sarcomas did not appear to benefit from whole-abdominal irradiation and that it may have reduced intra-abdominal recurrence in only a small subset of patients. We have used whole-abdominal radiation in patients deemed at risk for intra-abdominal metastatic disease. In our series, we used whole-abdominal therapy in patients with advanced stage or serous histology or both. With a median follow-up of 2.1 years, the 5-year relapse-free survival rate was 70% with a 5-year actuarial overall survival of 86%.92 In our series, with a conservative whole-abdominal radiation therapy dose and selected para-aortic nodal boost, no significant toxicity was noted.
The GOG also performed a prospective, phase II trial of whole-abdominal radiation therapy in stage III and IV maximally debulked patients. The 3-year survival was 31%.93 Smith and associates,94 in an update of the Stanford experience, noted a 3-year disease-free survival rate of 79% with an overall survival rate of 89% in patients with stage III or IV endometrial adenocarcinoma.
Chemotherapy
A phase II study was conducted by the Radiation Therapy Oncology Group (RTOG 9708) combining adjuvant pelvic radiation therapy with concomitant chemotherapy followed by chemotherapy in grade 2 or 3 endometrial adenocarcinoma with either >50% myometrial invasion, cervical stromal invasion, or pelvic-confined extra-uterine disease. Forty-six patients were enrolled with a median follow-up time of 4.3 years. Chronic toxicity was grade 1 in 16%, grade 2 in 41%, grade 3 in 16%, and grade 4 in 5%. Overall survival and disease-free survival were 85% and 81%, respectively. The 4-year pelvic, regional, and distant recurrence rates were 2%, 2%, and 19%, respectively. There were no recurrences in patients with stage IC, IIA, or IIB disease. While patients with extrauterine stage III disease demonstrated a pattern of distant recurrence, this trial illustrates the potential of combined therapy in the postoperative treatment for patients with disease confined to the uterus.95
A randomized phase III study in early stage high risk endometrial cancer patients compared adjuvant radiation therapy with or without chemotherapy (NSGO-EC-9501/EORTC 55991). Eligible patients had surgical stage I, II, IIA (with positive peritoneal cytology only), or IIIC (positive pelvic lymph nodes only) and qualified for adjuvant therapy based on risk of micrometastatic disease. Radiation therapy was external beam radiation therapy to 44 Gy with or without a vaginal brachytherapy boost. Chemotherapy consisted of several regimens: cisplatin + doxorubicin or epirubicin for four courses (AP), paclitaxel + epirubicin + carboplatin AUC 5, and paclitaxel + carboplatin AUC 5-6. In all 367 patients were evaluable with a median follow up of 3.5 years. The hazard ratio for progression free survival was 0.58 in favor of combined therapy (p = 0.046), which translated into an estimated 7% absolute difference in progression free survival from 75 to 82%.96
The GOG 122 trial randomized patients between whole abdominal radiation therapy and chemotherapy with cisplatin and doxorubicin. A total of 396 patients with stage III or IV endometrial cancer were randomized to receive whole-abdominal irradiation (30 Gy in 20 fractions, with a 15 Gy boost) or chemotherapy with doxorubicin and cisplatin every 3 weeks for seven cycles, followed by one cycle of cisplatin. With a median follow up of 74 months, the hazard ratio for progession of disease was 0.74 favoring the chemotherapy arm. The stage-adjusted death hazard ratio was 0.68, also favoring the chemotherapy group.97
Mundt and coworkers98 reported recurrence rates in 43 patients with stage I–IV endometrial cancer who received adjuvant chemotherapy alone. A recurrence rate of 67.4% was seen, with a 3-year actuarial pelvic recurrence rate of 48.1%. Thirty-one per cent of recurrent patients recurred in the pelvis alone. Given these results, adjuvant chemotherapy protocols in endometrial cancer should probably continue to incorporate locoregional radiation therapy.
UTERINE PAPILLARY SEROUS CARCINOMA
As discussed previously, patients with uterine papillary serous carcinoma have a higher recurrence rate compared to those with other uterine adenocarcinomas; there is also a preponderance of upper abdominal failures in these patients.17, 31, 32, 33, 34, 35, 36, 37 This has led a number of investigators to attempt more aggressive adjuvant radiation therapy, including whole-abdominal irradiation.
Published reports of studies using whole-abdominal irradiation in patients with uterine papillary serous carcinoma suggest a reduction in recurrence rates in early stage disease. Mallipeddi and associates99 reported the use of whole-abdominal radiation on ten patients with uterine papillary serous carcinoma, five of whom were alive at follow-up. This study noted long-term control in patients with superficial myometrial invasion, with or without positive cytology, who received optimal radiation. As in a previous report,100 vaginal recurrences were lower with a vaginal-cuff boost. Gibbons and coworkers88 noted a 60% long-term recurrence-free survival in a group of patients who received whole-abdominal irradiation therapy. We noted an excellent 86% 5-year actuarial survival in our patients treated with whole-abdominal radiation therapy.92 This is in contrast to the low 3-year survival in GOG-94.93, 101, 102 Chemotherapy is also being utilized frequently as an adjuvant therapy for papillary serous cancer.
Table 2 reviews various series using whole-abdominal radiation.
Table 2. Clinical results of whole-abdominal radiation
Reference | No. of Patients | % Serous Histology | Survival (%) | Recurrence Rate (%) | Follow-Up (Median Months) |
Mallipeddi et al.99 | 10 | 100 | 60 | 50 | 64 |
Frank et al.100 | 9 | 100 | 55 | 67 | 25 |
Greer and Hamberger89 | 31 | 63* (5 year) | 19 | >24 | |
Gibbons et al.88 | 56 | 18 | 64 (7 year) | 36 | 45 |
Loeffler et al.91 | 16 | 50 (1.5 year) | 62.5 | 17 | |
Axelrod et al.93 | 77 | 0 | 31 (3 year) | ||
Axelrod et al.93 | 88 | NS | 33 (3 year) | ||
Small et al.92 | 30 | 47 | 86 (5 year) | 23 | 27 |
Potish et al.90 | 27 | 0 | 71 | 25 | NS |
Smith et al.94 | 48 | NS | 77 (3 year) | 40 | 37 (mean) |
*For patients with residual disease <2 cm (n = 27).
NS, not significant.
TECHNIQUES OF RADIATION THERAPY
Radiation can be delivered by means of external sources (EBRT), implanted irradiation (brachytherapy), or radioactive fluid. This section discusses EBRT and brachytherapy. Radioactive fluid instillation is occasionally done intraperitoneally with P37most commonly as adjuvant therapy in ovarian cancer. Some work has been done using P37 in patients with endometrial carcinoma with positive cytology;103 this work is not discussed further, however, because data are somewhat limited.
EBRT is used to irradiate areas thought to be at risk for disease recurrence, including the whole pelvis, the whole pelvis plus the para-aortic nodal region, and the whole abdomen. EBRT is produced by cobalt machines, linear accelerators, or with charged particle cyclotrons (i.e., protons). As the energy of radiation increases, the beam penetration also increases, making it possible to limit the peripheral radiation needed for delivery of a desired dose at depth. Because the pelvis has a relatively thick separation, higher energy beams are preferred. There are limited data regarding charged particle therapy and this form of therapy is beyond the scope of this chapter.
Whole-abdominal irradiation is used to irradiate the entire abdominal contents. With modern radiation machines, this usually can be accomplished with a single setup, treating with an anterior and posterior field. The total whole-abdominal dosage is usually limited to 2000–3000 cGy in fractions of 100–150 cGy per treatment. Vital organs may need to be shielded to limit the radiation dose. The kidneys should be shielded to limit the dose to approximately 1800 cGy; liver shielding should also be considered if the dose exceeds 2500 cGy. Whole-abdominal irradiation in endometrial cancer is usually followed by a boost to the pelvis, preceded in many situations by a para-aortic nodal boost.
Treatment of the para-aortic nodes can be accomplished with either separate fields matched to the pelvic field or in continuity with pelvic radiation fields. We prefer to use a single field to avoid problems of matching. The para-aortic nodes can be treated with a two-field or four-field technique, generally to a total dosage of 4500 cGy at 180 cGy per fraction. If a two-field technique is used, care must be taken to ensure that the dose to the spinal cord is limited to less than 4500 cGy. If a four-field technique is used, the location of the kidneys must be verified to avoid exceeding kidney tolerance.
Whole-pelvic irradiation can be accomplished by either a two-field or four-field technique using 3D conformal radiation therapy or with intensity modulated radiation therapy. To avoid excessive maximal dosages, the two-field technique should be used only with high-energy beams. The two-field technique uses opposed anterior and posterior fields. The upper border of the field is generally placed at the L4-5 or L5-S1 interspace. If there is no disease extension into the vagina, the lower border should encompass one half to two thirds of the vagina. The lateral borders should be placed approximately 1.5 cm lateral to the bony pelvic rim. A marker should always be placed to indicate the location of the vaginal cuff/cervix or the most distal aspect of tumor extension. The four-field technique allows lateral shielding of structures that cannot be shielded in the anteroposterior field. This is our preferred method of pelvic irradiation. In the four-field technique, the upper and lower field borders are identical to those in the two-field technique. The anterior border of the lateral field is placed at or anterior to the anterior pubic symphysis. The posterior border is placed at the S2-3 interspace unless tumor extension necessitates larger fields. With 3D conformal radiation therapy, currently the standard of care for radiation therapy, the clinical target volume, defined as the area which is at risk for harboring microscopic metastatic disease, is outlined on a CT scan. Normal tissues, such as bladder, rectum, large intestine, and small intestine, are also outlined in the same manner. Anteroposterior, posteroanterior, and lateral field borders are defined to include the clinical target volume while sparing as much normal tissue as possible. A dose volume histogram, or DVH, can then be created to define the amount of normal tissue receiving a certain critical dose if felt to be clinically important.
Pelvic radiation therapy technique is extremely important in treatment outcomes, especially in reducing short-term and long-term toxicity.104 Barium should be given at the time of simulation to document the position of the small bowel.105 Attempts to reduce the small bowel in the radiation field include placing the patient in the prone position with a full bladder with or without abdominal compression. Patients should always be treated with a full bladder to move as much of the small bowel as possible out of the pelvic field. The total pelvic radiation therapy dosage typically is 45–50 Gy for adjuvant therapy.
Intensity modulated radiation therapy (IMRT) is a radiation technique which is currently being investigated for treatment of gynecologic malignancies including endometrial cancer. This technique allows for decreased radiation doses to critical structures such as bone marrow or small bowel while continuing to treat the tumor to the same dose. Several small trials have showed an improved toxicity profile with IMRT.106, 107, 108 A recently closed trial, RTOG 0418, was designed to assess the utility, efficacy, side effects, and control and survival rates when IMRT is used for postoperative endometrial and cervical cancer.
Brachytherapy refers to the placement of a radioactive source in or near the desired treatment volume. This allows a higher local radiation dose and spares surrounding normal tissues. The two main forms of delivery of brachytherapy are the LDR and the HDR techniques. The LDR technique uses isotopes that deliver radiation with a dose rate of approximately 40–100 cGy/h to the prescribed target. HDR brachytherapy, which delivers approximately 200 cGy/min, can be performed on an outpatient basis. There is a significant biologic difference between LDR and HDR brachytherapy: HDR delivery has a higher “effective” radiation dose for the same nominal LDR dose. Therefore, the delivered HDR doses must be adjusted lower to give the same effective LDR treatment.
The isotopes used in LDR treatment typically include cesium-137 or radium-226. Radium-226 has fallen out of favor because of radiation safety issues. Cesium-137 has a half-life of 30 years, allowing reuse of a source over a long period, although periodic calibration to allow for decay is necessary. HDR treatments typically use an iridium-192 source that needs frequent recalibration and replacement. Iridium-192 can also be used as an LDR isotope.
Typically, in most gynecologic applications of brachytherapy, the sources of radiation are left in place temporarily and then removed. This is the case in most LDR applications and all HDR applications. Permanent LDR brachytherapy procedures have a limited use in gynecologic malignancies and are not discussed further.
The sources of radiation are, in almost every case, afterloaded into a hollow radiation carrier. This permits some planning before determining the strength of radioactive isotope to use and significantly reduces radiation exposure during placement. The carriers used for afterloading can be divided roughly into those used to treat the intact uterus and those used after surgery.
The uterus may be treated with a tandem alone, as is done in treating cervical cancer. Treating the uterus with a tandem alone may underdose the thicker sections of the myometrium. The use of duel curved tandems, as noted above, has been shown to have good outcome data for toxicity, recurrence, and survival for unresectable disease.109 Heyman15 originally described using multiple radium capsules packed into the uterus to stretch and thin the wall to improve the dose distribution. Simon and Silverstone110 later developed afterloading capsules to decrease radiation exposure during placement.
Brachytherapy dose is defined either in terms of actual dosage delivered or in terms of total milligram-hours, which is simply derived by multiplying the total milligrams of equivalent radium by the total number of hours of the implant. The doses of radiation used when delivered before surgery with planned hysterectomy typically range from 2500 to 4000 mg-h to the uterus using a tandem or Simon-Heyman capsules and colpostats to deliver 1900–2000 mg-h (6000–6500 cGy vaginal surface dose) to the upper vagina. In some patients, 50 Gy of postoperative EBRT is added, with the whole-pelvic dosage limited to approximately 2000 cGy by the addition of a midline shield. When definitive radiation is delivered without planned hysterectomy, uterine milligram-hours range from 3000 to 10,000, depending on whether EBRT is also delivered.1, 2, 3, 4, 5, 6 Although not commonly reported, the point A dose (i.e., the dose defined as 2 cm superior and 2 cm lateral to the external os) is approximately 7500–8500 cGy.1, 3 HDR is generally delivered in a fractionated manner, with an attempt to deliver biologically equivalent dose to the traditional LDR implants.
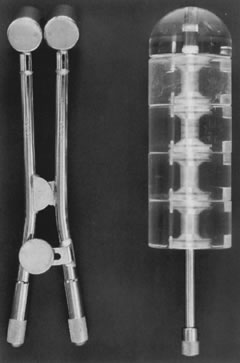
Posthysterectomy vaginal brachytherapy is generally delivered with a vaginal cylinder or with ovoids (Fig. 1). The dose delivered with low-dose brachytherapy alone tends to be prescribed at the vaginal surface. Doses range from 6000 to 7000 cGy.42, 47, 48 The use of postoperative high-dose brachytherapy is becoming more common, allowing outpatient treatment. A common dose schedule is 2100 cGy divided into three fractions of 700 cGy and prescribed to 0.5 cm from the vaginal mucosal surface.46 There is quite a bit of variation in the dose schedule for high dose rate radiation amongst radiation oncologists.111
|
The ideal timing of postoperative radiation therapy is not known. There is support for initiating postoperative irradiation within 6 weeks after surgery. A higher local failure rate was seen with a delay of longer than 6 weeks.112 Given the time needed to initiate treatment planning, patients for whom postoperative irradiation is being considered should be referred immediately to the radiation oncologist to prevent nonmedical delays in the initiation of therapy.
The need for a vaginal-cuff boost after postoperative EBRT recently has been questioned by a number of investigators.112, 113 We use a vaginal cuff boost in selected patients. Numerous large studies have consistently used vaginal-cuff boosts with excellent long-term results.45, 46 In addition, at least one nonrandomly assigned review noted improved local control with the addition of a vaginal-cuff boost to postoperative EBRT.114 The number of absolute vaginal-cuff recurrences prevented by a vaginal-cuff boost is probably small. We utilize HDR vaginal-cuff boosts and have used a 600 cGy vaginal surface dose for two to three fractions. Other institutions deliver a higher total vaginal mucosal dose and limit the midpelvis external dose by using a midline shield.45
RECURRENT DISEASE
Locoregionally recurrent endometrial cancer can be cured with radiation therapy. Results tend to be best in patients with vaginal-cuff recurrences and without previous irradiation. Curran and colleagues115 reported on 55 patients with isolated vaginal recurrences who were treated with definitive irradiation. Patients with vaginal mucosal recurrence had a 3-year actuarial survival and a local control rate of 85% and 100%, respectively. This compared with a 13% 3-year actuarial survival and a 0% local control rate in patients with sidewall involvement at the time of recurrence. The 5-year survival rate overall was 48% in patients who did not receive previous irradiation compared to 16% in patients who were receiving their second course of radiation therapy. Other authors have seen similar results.116, 117, 118, 119, 120, 121 The PORTEC trial also noted a 3-year survival of 69% after vaginal recurrence compared to 13% after pelvic or distant relapse.56 Other prognostic factors noted included histologic type of recurrence,118 time to recurrence,117 and tumor size.121
The exact technique used in salvage irradiation needs to be individualized for each patient. Generally, a combination of EBRT and brachytherapy should be used. Because recurrent disease is not confined by the normal anatomic barrier of the uterus, EBRT to sterilize nonpalpable disease should probably always be part of the planned therapy. There are several current Gynecologic Oncology Group trials which are investigating chemotherapy agents for recurrent endometrial cancer. For instance, in GOG 0209, cisplatin, doxorubicin, paclitaxel, and filgrastim is being compared to carboplatin and paclitaxel in patients with stage III, IV, or recurrent endometrial cancer. Protocol UMN-2004LS021 is investigating induction chemotherapy with carboplatin and docetaxel followed by radiation therapy and consolidation chemotherapy with the same agents in patients with stage III, IV, or recurrent endometrial cancer.
UTERINE SARCOMAS
Uterine sarcomas tend to behave in a more malignant fashion than do endometrial cancers. The three most common histologic variants of uterine sarcomas are endometrial stromal sarcoma (ESS), leiomyosarcoma (LMS), and carcinosarcoma.
As in endometrial adenocarcinomas, surgery is the preferred primary therapy in uterine sarcomas.
A number of institutions have reviewed their experience in patients who have received adjuvant radiation and compared the results to patients who underwent surgery alone. The data are presented in some series as uterine sarcomas, and in others the histologies are divided among carcinosarcoma, LMS, and ESS. Given the selective use of radiation in these trials, a bias toward irradiating patients with poor prognostic features would be expected. Despite this bias, there is significant evidence to support the use of adjuvant radiation in many patients. There seems to be a general consensus that postoperative radiation therapy improves local control in carcinosarcomas.122, 123, 124, 125, 126, 127 Some reviews support an improvement in survival,122, 123, 125, 126, 127, 128 while others do not.129, 124
A randomized trial was recently reported in which 224 patients who underwent TAH-BSO and were randomized to adjuvant EBRT (50.4 Gy in 28 fractions) or no further therapy. Results showed improved locoregional control with adjuvant EBRT (p = 0.004), but did not show an improved overall survival or progression-free survival.130
LMSs tend to have a higher propensity for distant metastasis, and it would, therefore, follow that local adjuvant treatments may have less of an influence on survival. There is evidence in some series for an improvement in local control with the addition of adjuvant radiation.131, 132 Hornback and coworkers,133 conversely, reviewing the use of radiation in GOG-20, did not find a difference in first recurrence rates with the use of adjuvant radiation in LMS, although an improvement in pelvic control was seen in the mixed mesodermal sarcomas. There is less support regarding survival improvements. At least one institution noted no improvement in survival for adjuvant radiation when treating LMSs with low mitotic activity.134
A recent GOG Phase III study135 examined the postoperative efficacy of post-operative whole abdominal irradiation (WAI) with 1 Gy BID or 1.5 Gy daily to a total dose of 30 Gy compared to adjuvant cisplatin, ifosfamide and mesna (CIM). The study focused on patients with carcinosarcoma and included all stages of disease.In all 232 patients were randomized with 43% of the patients being stage I–II, and 45% stage III. There were a total of 112 recurrences, with 60 occurring in the WAI group and 52 in the CIM group. There were no significant differences in the number or site of recurrences. However, there were slightly more vaginal recurrences with CIM and slightly more abdominal recurrences with WAI. There was no significant survival difference.
Endometrial stromal sarcomas have traditionally been divided into low grade and high grade. The NCCN guidelines have modified the classification of endometrial stromal sarcomas, so that the group previously referred to as high-grade endometrial stromal sarcomas is now known as high-grade undifferentiated sarcomas, and the group previously referred to as low- grade endometrial stromal sarcomas is now known simply as endometrial stromal sarcoma.136 Patients with low-grade endometrial stromal sarcoma tend to have a favorable prognosis, and there is little evidence that in early stage disease, adjuvant radiation would offer a benefit.137 There is evidence that adjuvant radiation may improve local control in patients with high-grade endometrial stromal sarcoma122, 128 and possibly survival.128, 138
In a series reported by Weitmann and associates139 of patients with ESS, a 93.8% 5-year local control rate was seen in patients who received surgery and radiation therapy, with the majority of patients having high-grade disease. The actuarial overall survival at 10 years was 52.8%.
A number of publications have looked at uterine sarcomas without dividing the patients into separate histologic categories. A report by the Grup Oncologic Catala-Occita reviewed their experience in 103 patients with uterine sarcomas. A local control and survival advantage was seen with adjuvant radiation.140 The Curie Institute also reported on uterine sarcomas and found an improvement in local control with the addition of radiation in high-grade tumors.141
Given the overall rarity of uterine sarcoma, the above discussion basically focuses on earlier stage disease. The use of adjuvant radiation in advanced disease is based on even more limited data and extrapolations from endometrial adenocarcinoma results. There is a need for open dialogue between patient and physician, and also for further studies investigating the role of multimodality therapy.
SEQUELAE OF THERAPY
In general, when surgery and adjuvant irradiation are combined, the incidence of severe complications appears to be less than 10% with proper technique. In a detailed analysis of 304 patients treated at the Mallinckrodt Institute, Stokes and associates142 noted an overall rate of serious complications of 4%, with none fatal. The highest complication rate was noted in patients who had been treated with a preoperative implant followed by postoperative EBRT. These investigators also noted a serious complication rate of only 1% when a preoperative implant alone was used. The complications seen included five bowel obstructions, one malabsorption syndrome, two rectal ulcers, one vaginal obliteration, one uterovaginal fistula, one chronic cystitis, and one urethral stricture with obstruction. Other authors have noted somewhat higher complication rates; these studies, however, included mild and moderate complications. Nori and coworkers46 noted a 9% mild and moderate complication rate, including cystitis (4.5%), vaginal stenosis (2.5%), proctitis (1.5%), vaginal vault necrosis (0.5%), and partial bowel obstruction (0.5%). No surgical intervention was necessary. Piver and colleagues44 noted a higher significant complication rate of 9.7% in their patients who received whole-pelvic irradiation. No significant complications were seen in their patients who had received vaginal radiation therapy only. The PORTEC trial group noted a 26% 5-year actuarial rate of late complications in the group who received radiation therapy and a 4% risk of complications in the group that received no postoperative therapy. The risk of grade 3–4 complications was 3% in the group who received postoperative radiation therapy and 0% in the other group.143 In summary, patients who are treated with vaginal radiation therapy alone have a very small risk of severe complications. The addition of EBRT increases the complication rate, although in most series, the rate is less than 5%.42, 46, 47, 51, 52, 142
Rates of severe radiation complications seen in patients who have received radiation alone are, in general, similar to the rates seen in patients who have received adjuvant treatment.1, 2, 3, 4, 5 Grigsby and coworkers6 noted a somewhat higher severe complication rate of 16%. Nguyen and associates,9 using HDR brachytherapy, noted an 11% serious complication rate. Of particular interest in patients with endometrial cancer treated with radiation alone is the risk of medical complications related to the brachytherapy procedure in these patients, who, for the most part, are medically inoperable. Chao and associates144 gave a detailed analysis of 150 LDR implants performed on 96 medically inoperable patients. There was a 4.2% morbidity rate and a 2.1% mortality rate (one myocardial infarction and one pulmonary embolism). Despite the predicted serious complication rate of surgery in these patients, the number of life-threatening brachytherapy-related complications appears to be reasonable.
CONCLUSION
Radiation plays a prominent role in the treatment of uterine tumors. Its most common role is in the adjuvant setting after hysterectomy. There may also be a role for adjuvant irradiation in some uterine sarcomas. When applied properly, radiation can contribute to tumor control with acceptable rates of serious complications.
REFERENCES
Lehoczky O, Bosze P, Ungar L et al:Stage I endometrial carcinoma: Treatment of nonoperable patients with intracavitary radiation therapy alone. Gynecol Oncol 43: 211, 1991 |
|
Kupelian PA, Eifel PJ, Tornos C et al:Treatment of endometrial carcinoma with radiation therapy alone. Int J Radial Oncol Biol Phys 27: 817, 1993 |
|
Rouanet P, DuBois JB, Gely S et al:Exclusive radiation therapy in endometrial carcinoma. Int J Radial Oncol Biol Phys 26: 223, 1993 |
|
Varia M, Rosenman L, Halle J et al:Primary radiation therapy for medically inoperable patients with endometrial carcinoma, stages I-II. Int J Radiat Oncol Biol Phys 13: 11, 1987 |
|
Fishman DA, Roberts KB, Chambers JT et al:Radiation therapy as exclusive treatment for medically inoperable patients with stage I and II endometrial carcinoma of the endometrium. Gynecol Oncol 61: 189, 1996 |
|
Grigsby PW, Kuske RR, Perez CA et al:Medically inoperable stage I adenocarcinoma of the endometrium treated with radiotherapy alone. Int J Radial Oncol Biol Phys 13: 483, 1987 |
|
Chao CKS, Grigsby PW, Perez CA et al:Medically inoperable stage I endometrial carcinoma: A few dilemmas in radiotherapeutic management. Int J Radial Oncol Biol Phys 34: 27, 1996 |
|
Patanaphan V, Salazar OM, Chougule P: What can be expected when radiation therapy becomes the only curative alternative for endometrial cancer? Cancer 55: 1462, 1985 |
|
Nguyen C, Souhami L, Roman TN et al:High-dose rate brachytherapy as the primary treatment of medically inoperable stage I-II endometrial carcinoma. Gynecol Oncol 59: 370, 1995 |
|
Landgren RC, Fletcher GH, Delclos L et al:Irradiation of endometrial cancer in patients with medical contraindications to surgery or with unresectable lesions. Am J Roentgenol 126: 148, 1976 |
|
Nguyen TV, Petereit DG: High-dose-rate brachytherapy for medically inoperable stage I endometrial cancer. Gynecol Oncol 71: 196, 1998 |
|
Knocke TH, Kucera H, Weidinger B et al:Primary treatment of endometrial carcinoma with high-dose-rate brachytherapy: Results of 12 years of experience with 280 patients. Int J Radial Oncol Biol Phys 37: 359, 1997 |
|
Rose PG, Baker S, Kern M et al:Primary radiation therapy for endometrial carcinoma: A case controlled study. Int J Radial Oncol Biol Phys 27: 585, 1993 |
|
Morrow CP, Bundy BN, Kurman RJ et al:Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol Oncol 40: 55, 1991 |
|
Heyman J: The so-called Stockholm method and results of treatment of uterine cancer at the Radiumhemmet. Acta Radiol 16: 129, 1935 |
|
Coon D, Beriwal S, Heron DE et al: High-dose-rate Rotte "Y" applicator brachytherapy for definitive treatment of Int J Radiat Oncol Biol Phys. 2008 Jul 1;71(3):779-83. Epub 2008 Feb 6. |
|
Eifel PJ, Ross J, Hendrickson M et al:Adenocarcinoma of the endometrium: Analysis of 256 cases with disease limited to the uterine corpus—Treatment comparisons. Cancer 52: 1026, 1983 |
|
Price L, Hohr GA, Rominjor DJ: Vaginal involvement in endometrial carcinoma. Am J Obstet Gyneco191:1060, 1965 |
|
Pettersson F: Annual report on the results of treatment in gynecologic cancer. Int Fed Gynecol Obstet 22: 36, 1994 |
|
Fanning J, Alvarez PM, Tsukada Y et al:Prognostic significance of cervical involvement by endometrial cancer. Gynecol Oncol 40: 46, 1991 |
|
Larson DM, Copeland LJ, Gallager HS et al:Nature of cervical involvement in endometrial carcinoma. Cancer 59: 959, 1987 |
|
Onsrud M, Aalders J, Abeler V et al:Endometrial carcinoma with cervical involvement (stage II): Prognostic factors and value of combined radiological-surgical treatment. Gynecol Oncol 13: 76, 1982 |
|
Wallin TC, Malkasian GD Jr, Gaffey TA et al:Stage II cancer of the endometrium: A pathologic and clinical study. Gynecol Oncol 18: 1, 1984 |
|
Tsuruchi N, Kaku T, Kamura T et al:The prognostic significance of lymphovascular space invasion in endometrial cancer when conventional hematoxylin and eosin staining is compared to immunohistochemical staining. Gynecol Oncol 57: 307, 1995 |
|
Sivridis E, Buckley CH, Fox H: The prognostic significance of lymphatic vascular space invasion in endometrial adenocarcinoma. Br J Obstet Gynaecol 94: 991, 1987 |
|
Hanson MB, Van Nagell JR, Powell DE et al:The prognostic significance of lymph-vascular space invasion in stage I endometrial cancer. Cancer 55: 1753, 1985 |
|
Zaino RJ, Kurman RJ, Diana KL et al: Pathologic models to predict outcome for women with endometrial adenocarcinoma:Gynecologic Oncology Group study. Cancer. 1996 Mar 15;77(6):1115-21. |
|
Lurain JR, Rice BL, Rademaker AW et al: Prognostic factors associated with recurrence in clinical stage I adenocarcinomaof the endometrium. Obstet Gynecol. 1991 Jul;78(1):63-9. |
|
Morrow CP, Bundy BN, Kurman RJ et al: Relationship between surgical-pathological risk factors and outcome in clinicalstage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991 Jan;40(1):55-65. |
|
Lurain JR, Rice BL, Rademaker AW et al: Prognostic factors associated with recurrence in clinical stage I adenocarcinomaof the endometrium. Obstet Gynecol. 1991 Jul;78(1):63-9. |
|
Jeffrey JF, Krepart GV, Lotocki PJ: Papillary serous adenocarcinoma of the endometrium. Obstet Gynecol 67: 670, 1986 |
|
Carcangiu ML, Chambers JT: Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium: Comparison of clinicopathologic features and survival. Int J Gynecol Pathol 14: 30, 1995 |
|
Rosenberg P, Blom R, Hogberg T et al:Death rate and recurrence pattern among 841 clinical stage I endometrial cancer patients with special reference to uterine papillary serous carcinoma. Gynecol Oncol 51: 311, 1993 |
|
Ward BG, Wright RG, Free K: Papillary carcinomas of the endometrium. Gynecol Oncol 39: 347, 1990 |
|
Sutton GP, Brill L, Michael H et al:Malignant papillary lesions of the endometrium. Gynecol Oncol 27: 294, 1987 |
|
Chapman GW: Papillary adenocarcinoma of the endometrium: A retrospective study of stage I and II disease. J Natl Med Assoc 86: 118, 1994 |
|
Gallion HH, Van Nagell JR, Powell DF et al:Stage I serous papillary carcinoma of the endometrium. Cancer 63: 2224, 1989 |
|
Christopherson WM, Alberhasky RC, Connelly PJ: Carcinoma of the endometrium: II. Papillary adenocarcinoma: A clinical pathological study of 46 cases. Am Soc Clin Pathol 77: 534, 1992 |
|
O'Hanlan KA, Levine PA, Harbatkin D et al:Virulence of papillary endometrial carcinoma. Gynecol Oncol 37: 112, 1990 |
|
Kurman RJ, Scully RE: Clear cell carcinoma of the endometrium. Cancer 37: 872, 1976 |
|
Abeler VM, Kiorstad KE: Clear cell carcinoma of the endometrium: A histopathological and clinical study of 97 cases. Gynecol Oncol 40: 207, 1991 |
|
Aalders J', Abeler V, Kolstad P et al:Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma. Obstet Gynecol 56: 419, 1980 |
|
Piver MS, Yazigi R, Blumenson L et al:A prospective trial comparing hysterectomy, hysterectomy plus vaginal radium, and uterine radium plus hysterectomy in stage I endometrial carcinoma. Obstet Gynecol 54: 85, 1979 |
|
Piver MS, Hempling RE: A prospective trial of postoperative vaginal radium/cesium for grade 1–2 less than 50% myometrial invasion and pelvic radiation therapy for grade 3 or deep myometrial invasion in surgical stage I endometrial adenocarcinoma. Cancer 66: 1133, 1990 |
|
Grigsby P, Perez CA, Kuten A et al:Clinical stage I endometrial cancer: Results of adjuvant irradiation and patterns of failure. Int J Radiat Oncol Biol Phys 21: 379, 1991 |
|
Nori D, Hilaris BS, Tome M et al:Combined surgery and radiation in endometrial carcinoma: An analysis of prognostic factors. Int J Radiat Oncol Biol Phys 13: 489, 1987 |
|
Mayr NA, Wen B-C, Benda JA et al:Postoperative radiation therapy in clinical stage I endometrial cancer: Corpus, cervical, and lower uterine segment involvement—patterns of failure. Radiology 196: 232, 1995 |
|
Huguenin PU, Glanzmann C, Hammer F et al:Endometrial carcinoma in patients aged 75 years or older: Outcome and complications after postoperative radiotherapy or radiotherapy alone. Strahlenther Onkol 168: 567, 1992 |
|
Boz G, De Paoli A, Innocente R et al:Endometrial stage I carcinoma treated with surgery and adjuvant irradiation: A retrospective analysis. Tumori 81: 256, 1995 |
|
Reddy S, Lee M-S, Hendrickson FS: Pattern of recurrences in endometrial carcinoma and their management. Radiology 133: 737, 1979 |
|
Seski J, Teneriello M, Kalnicki S: A retrospective review of postoperative radiation therapy in patients with endometrial carcinoma (abstr). Gynecol Oncol 60: 97, 1996 |
|
Petereit DG, Tannehill JC, Schink BR et al:Adjuvant high dose rate vaginal cuff brachytherapy for early-stage endometrial carcinoma (abstr). Gynecol Oncol 60: 97, 1996 |
|
Gretz HF, Economos K, Husain A et al:The practice of surgical staging and its impact on adjuvant treatment recommendations in patients with stage I endometrial carcinoma. Gynecol Oncol 61: 409, 1996 |
|
Roberts JA, Brunetto VL, Keys HM et al:A phase III randomly assigned study of surgery vs surgery plus adjunctive radiation therapy in intermediate-risk endometrial adenocarcinoma (GOG No. 99). Gynecol Oncol 68: 135, 1998 |
|
Keys HM, Roberts JA, Brunetto VL et al: A phase III trial of surgery with or without adjunctive external pelvic radiationtherapy in intermediate risk endometrial adenocarcinoma: a Gynecologic OncologyGroup study. Gynecol Oncol. 2004 Mar;92(3):744-51. |
|
Creutzberg CL, van Putten WLJ, Koper PCM et al:Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. Lancet 355: 1404, 2000 |
|
Scholten AN, van Putten WL, Beerman H et al: Postoperative radiotherapy for Stage 1 endometrial carcinoma: long-term outcomeof the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys. 2005 Nov 1;63(3):834-8. Epub 2005 May 31. |
|
Chadha M, Nanavati PJ, Liu P et al: Patterns of failure in endometrial carcinoma stage IB grade 3 and IC patientstreated with postoperative vaginal vault brachytherapy. Gynecol Oncol. 1999 Oct;75(1):103-7. |
|
Pearcey RG, Petereit DG: Post-operative high dose rate brachytherapy in patients with low to intermediaterisk endometrial cancer. Radiother Oncol. 2000 Jul;56(1):17-22. |
|
Anderson JM, Stea B, Hallum AV et al: High-dose-rate postoperative vaginal cuff irradiation alone for stage IB and ICendometrial cancer. Int J Radiat Oncol Biol Phys. 2000 Jan 15;46(2):417-25. |
|
Eltabbakh GH, Piver MS, Hempling RE et al: Excellent long-term survival and absence of vaginal recurrences in 332 patientsvaginal brachytherapy without formal staging lymph node sampling: report of aprospective trial. Int J Radiat Oncol Biol Phys. 1997 May 1;38(2):373-80. |
|
Nori D, Merimsky O, Batata M et al: Postoperative high dose-rate intravaginal brachytherapy combined with external Int J Radiat Oncol Biol Phys. 1994 Nov 15;30(4):831-7. |
|
Solhjem MC, Petersen IA, Haddock MG: Vaginal brachytherapy alone is sufficient adjuvant treatment of surgical stage Iendometrial cancer. Int J Radiat Oncol Biol Phys. 2005 Aug 1;62(5):1379-84. |
|
Jolly S, Vargas C, Kumar T et al: Vaginal brachytherapy alone: an alternative to adjuvant whole pelvis radiationfor early stage endometrial cancer. Gynecol Oncol. 2005 Jun;97(3):887-92. |
|
J. Orton, P. Blake, on behalf of ASTEC/EN.5 collaborators. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement), 2007: 5504. |
|
R. A. Nout, H. Putter, I. M. Jürgenliemk-Schulz et al. Vaginal brachytherapy versus external beam pelvic radiotherapy for high-intermediate risk endometrial cancer: Results of the randomized PORTEC-2 trial. J Clin Oncol 26: 2008 (May 20 suppl; abstr LBA5503) |
|
Surwit EA, Fowler WC, Rogoff EE et al:Stage II carcinoma of the endometrium. Int J Radiat Oncol Biol Phys 5: 323, 1979 |
|
Homesley HD, Boronow RC, Lewis JL Jr: Stage II endometrial adenocarcinoma: Memorial Hospital for Cancer, 1949-1965. Obstet Gynecol 49: 604, 1977 |
|
Bruckman JE, Goodman RL, Murthy A et al:Combined irradiation and surgery in the treatment of stage II carcinoma of the endometrium. Cancer 42: 1146, 1978 |
|
Gagnon JD, Moss WT, Gabourel LS et al:External irradiation in the management of stage II endometrial carcinoma: A logical approach. Cancer 44: 1247, 1979 |
|
Grigsby PW, Perez CA, Camel HM et al:Stage II carcinoma of the endometrium: Results of therapy and prognostic factors. Int J Radiat Oncol Biol Phys 11: 1915, 1985 |
|
Maruyama Y, Yoneda J, Coffey C et al:Short communication: Tandem-vaginal cylinder applicator for radiation therapy of uterine adenocarcinoma. Radiother Oncol 25: 140, 1992 |
|
Reisinger SA, Staros EB, Feld R et al:Preoperative radiation therapy in clinical stage II endometrial carcinoma. Obstet Gynecol 45: 174, 1992 |
|
Boente MP, Orandi YA, Yordan EL et al:Recurrence patterns and complications in endometrial adenocarcinoma with cervical involvement. Ann Surg 2: 138, 1995 |
|
Greven K, Olds W: Radiotherapy in the management of endometrial carcinoma with cervical involvement. Cancer 60: 1737, 1987 |
|
Carl UM, Bahnsen J, Edel B et al:The value of postoperative radiation therapy in FIGO stage I and II endometrial cancers. Strahlenther Onkol 171: 322, 1995 |
|
Haenggi W, Gasser A, Feyereisl J et al:Curative treatment of clinical stage II endometrial carcinoma. Zentralbl Gynakol 117: 207, 1995 |
|
Cox JD, Komaki R, Wilson F et al:Locally advanced adenocarcinoma of the endometrium. Cancer 45: 715, 1980 |
|
Aalders JG, Abeler V, Kolstad P: Clinical (stage III) as compared to subclinical intrapelvic extrauterine tumor spread in endometrial carcinoma: A clinical and histopathological study of 175 patients. Gynecol Oncol 17: 64, 1984 |
|
Mackillop WJ, Pringle JF: Stage III endometrial carcinoma. Cancer 56: 2519, 1985 |
|
Danoff BF, McDay J, Louka M et al:Stage III endometrial carcinoma: Analysis of patterns of failure and therapeutic implications. Int J Radiat Oncol Biol Phys 6: 1491, 1980 |
|
Genest P, Drouin P, Girard A et al:Stage III carcinoma of the endometrium: A review of 41 cases. Gynecol Oncol 26: 77, 1987 |
|
Potish RA, Twiggs LB, Adcock LL et al:Paraaortic lymph node radiotherapy in cancer of the uterine corpus. Obstet Gynecol 65: 251, 1985 |
|
Delclos L, Fletcher GH, Gutierrez AG et al:Adenocarcinoma of the uterus. Am J Roentgenol 103: 603, 1969 |
|
Rose PG, Cba SD, Tak WK et al:Radiation therapy for surgically proven para-aortic node metastasis in endometrial carcinoma. Int J Radiat Oncol Biol Phys 24: 229, 1992 |
|
Fenn GA, Calerog A: Endometrial carcinoma: Treatment of positive para-aortic nodes. Gynecol Oncol 27: 104, 1987 |
|
Komaki R, Mattingly RF, Hoffman RG et al:Irradiation of para-aortic lymph node metastases, from carcinoma of the cervix or endometrium. Radiology 147: 245, 1983 |
|
Gibbons S, Martinez A, Schray M et al:Adjuvant whole abdominopelvic irradiation for high risk endometrial carcinoma. Int J Radiat Oncol Biol Phys 21: 1019, 1991 |
|
Greer BE, Hamberger AD: Treatment of intraperitoneal metastatic adenocarcinoma of the endometrium by the whole-abdomen moving-strip technique and pelvic boost irradiation. Gynecol Oncol 16: 365, 1983 |
|
Potish RA, Twiggs LB, Adcock LL et al:Role of whole abdominal radiation therapy in the management of endometrial cancer: Prognostic importance of factors indicating peritoneal metastases. Gynecol Oncol 21: 80, 1985 |
|
Loeffler JS, Rosen EM, Niloff JM et al:Whole abdominal irradiation for tumors of the uterine corpus. Cancer 61: 1332, 1988 |
|
Small W, Mahadevan A, Roland P et al:Whole-abdominal radiation in endometrial carcinoma: An analysis of toxicity, patterns of recurrence, and survival. Cancer 6: 394, 2000 |
|
Axelrod J, Bundy B, Roy T et al: Advanced endometrial carcinoma (EC) treated with whole abdominal irradiation (WAI): A Gynecologic Oncology Group (GOG) Study (abstr). Proceedings of the Society for Gynecologic Oncology, San Francisco, 1995:120 |
|
Smith RS, Kapp DS, Chen Q et al:Treatment of high-risk uterine cancer with whole abdominopelvic radiation therapy. Int J Radiat Oncol Biol Phys 48: 767, 2000 |
|
Greven K, Winter K, Underhill K et al: Final analysis of RTOG 9708: adjuvant postoperative irradiation combined withendometrial cancer. Gynecol Oncol. 2006 Oct;103(1):155-9. Epub 2006 Mar 20. |
|
Hogberg, P. Rosenberg, G. Kristensen, C. F. de Oliveira, R. de Pont Christensen, B. Sorbe, C. Lundgren, T. Salmi, H. Andersson, and N. S. Reed A randomized phase-III study on adjuvant treatment with radiation (RT) ± chemotherapy (CT) in early-stage high-risk endometrial cancer (NSGO-EC-9501/EORTC 55991) J Clin Oncol (Meeting Abstracts) 2007 25: 5503. |
|
Randall ME, Filiaci VL, Muss H et al: Randomized phase III trial of whole-abdominal irradiation versus doxorubicin andcisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic OncologyGroup Study. J Clin Oncol. 2006 Jan 1;24(1):36-44. Epub 2005 Dec 5. |
|
Mundt AJ, McBridge RB, Connell PP: Pelvic recurrence in high-risk pathologic stage I–IV endometrial carcinoma patients following adjuvant chemotherapy alone: Implications for locoregional radiation therapy. Int J Radiat Oncol Biol Phys 48: 124, 2000 |
|
Mallipeddi P, Kapp DS, Teng NNH: Long-term survival with adjuvant whole abdominopelvic irradiation for uterine papillary serous carcinoma. Cancer 71: 3076, 1993 |
|
Frank AH, Tsong PC, Haffty BG et al:Adjuvant whole abdominal radiation in uterine papillary serous carcinoma. Cancer 68: 1516, 1991 |
|
Sutton G, Axelrod JH, Bundy BN et al: Whole abdominal radiotherapy in the adjuvant treatment of patients with stage IIIand IV endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. 2005 Jun;97(3):755-63. |
|
Sutton G, Axelrod JH, Bundy BN et al: Adjuvant whole abdominal irradiation in clinical stages I and II papillary serousor clear cell carcinoma of the endometrium: a phase II study of the GynecologicOncology Group. Gynecol Oncol. 2006 Feb;100(2):349-54. Epub 2005 Oct 5. |
|
Soper JT, Creasman WT, Clarke-Person DL et al:Intraperitoneal chronic phosphate 3:p suspension therapy of malignant peritoneal cytology in endometrial carcinoma. Am J Obstet Gynecol 153: 191, 1985 |
|
Corn BW, Lanciano RM, Greven KM et al:Impact of improved irradiation technique, age, and lymph node sampling on the severe complication rate of surgically staged endometrial cancer patients: A multivariate analysis. J Clin Oncol 12: 510, 1994 |
|
Herbert SH, Curran WJ, Solin LJ et al:Decreasing gastrointestinal morbidity with the use of small bowel contrast during treatment planning for pelvic irradiation. Int J Radial Oncol Biol Phys 20: 235, 1991 |
|
Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 52:1330-7, 2002. |
|
Portelance L, Chao KS, Grigsby PW, et al. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys. 51:261-6, 2001. |
|
Roeske JC, Lujan A, Rotmensch J, et al. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 48:1613-21, 2000. |
|
Coon D, Beriwal S, Heron DE et al: High-dose-rate Rotte "Y" applicator brachytherapy for definitive treatment of Int J Radiat Oncol Biol Phys. 2008 Jul 1;71(3):779-83. Epub 2008 Feb 6. |
|
Simon N, Silverstone SM: Intracavitary radiotherapy of endometrial cancer by afterloading. Gynecol Oncol 1: 13, 1972 |
|
Small W Jr, Erickson B, Kwakwa F: American Brachytherapy Society survey regarding practice patterns ofpostoperative irradiation for endometrial cancer: current status of vaginalbrachytherapy. Int J Radiat Oncol Biol Phys. 2005 Dec 1;63(5):1502-7. Epub 2005 Aug 18. |
|
Ahmad NR, Lanciano RM, Corn BW et al:Postoperative radiation therapy for surgically staged endometrial cancer: Impact of time factors (overall treatment time and surgery-to-radiation interval) on outcome. Int J Radiat Oncol Biol Phys 33: 837, 1995 |
|
Torrisi JR, Barnes WA, Popescu G et al:Postoperative adjuvant external-beam radiotherapy in surgical stage I endometrial carcinoma. Cancer 64: 1414, 1989 |
|
Bliss P, Cowie VJ: Endometrial carcinoma: Does the addition of intracavitary vault caesium to external beam therapy postoperatively result in improved control or increased morbidity? Clin Oncol 4: 373, 1992 |
|
Curran WJ Jr, Whittington R, Peters AJ et al:Vaginal recurrences of endometrial carcinoma: The prognostic value of staging by a primary vaginal carcinoma system. Int J Radiat Oncol Biol Phys 15: 803, 1988 |
|
Aalders JG, Abeler V, Kolstad P: Recurrent adenocarcinoma of the endometrium: A clinical and histopathological study of 379 patients. Gynecol Oncol 17: 85, 1984 |
|
Kuten A, Grigsby PW, Perez CA et al:Results of radiotherapy in recurrent endometrial carcinoma: A retrospective analysis of 51 patients. Int J Radiat Oncol Biol Phys 17: 29, 1989 |
|
Mandell LR, Nori D, Hilaris B: Recurrent stage I endometrial carcinoma: Results of treatment and prognostic factors. Int J Radiat Oncol Biol Phys 11: 1103, 1985 |
|
Morgan JD, Reddy S, Sarin P et al:Isolated vaginal recurrences of endometrial carcinoma. Radiology 189: 609, 1993 |
|
Poulsen MG, Roberts S J: The salvage of recurrent endometrial carcinoma in the vagina and pelvis. Int J Radiat Oncol Biol Phys 15: 809, 1988 |
|
Sears JD, Greven KM, Hoen HM et al:Prognostic factors and treatment outcome for patients with locally recurrent endometrial cancer. Cancer 74: 1303, 1994 |
|
George M, Pejovic MH, Kramar A et al:Uterine sarcomas: Prognostic factors and treatment modalities—study on 209 patients. Gynecol Oncol 24: 58, 1986 |
|
Gerszten K, Faul C, Kounelis S et al:The impact of adjuvant radiotherapy on carcinosarcoma of the uterus. Gynecol Oncol 68: 8, 1998 |
|
Kohorn E, Schwartz P, Chambers J et al:Adjuvant therapy in mixed mullerian tumors of the uterus. Gynecol Oncol 23: 212, 1986 |
|
Larson B, Silfversward C, Nilsson B et al:Mixed mullerian tumours of the uterus—prognostic factors: A clinical and histopathologic study of 147 cases. Radiother Oncol 17: 123, 1990 |
|
Moskovic E, MacSweeney E, Law M et al:Survival, patterns of spread and prognostic factors in uterine sarcoma: A study of 76 patients. Br J Radiol 66: 1009, 1993 |
|
Vongtama V, Karlan J, Piver S et al:Treatment, results and prognostic factors in stage I and II sarcomas of the corpus uteri. Am J Roentgenol 126: 139, 1976 |
|
Brooks SE, Zhan M, Cote T et al: Surveillance, epidemiology, and end results analysis of 2677 cases of uterine Gynecol Oncol. 2004 Apr;93(1):204-8. |
|
Chi DS, Mychalczak B, Saigo PE et al:The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcoma. Gynecol Oncol 65: 493, 1997 |
|
Reed NS, Mangioni C, Malmstrom H et al: Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapyin the treatment of uterine sarcomas stages I and II: an European Organisationfor Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol55874). Eur J Cancer. 2008 Apr;44(6):808-18. Epub 2008 Apr 2. |
|
Ayhan A, Tuncer ZS, Tanir M et al:Uterine sarcoma: The Hacettepe hospital experience of 88 consecutive patients. Eur J Gynae Oncol 18: 146, 1997 |
|
Echt G, Jepson J, Steel J et al:Treatment of uterine sarcomas. Cancer 66: 35, 1990 |
|
Hornback NB, Omura G, Major FJ: Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radial Oncol Biol Phys 12: 2127, 1986 |
|
Nickie-Psikuta M, Gawrychowski K: Different types and different prognosis—study of 310 uterine sarcomas. Eur J Gynaec Oncol 14 (Suppl): 105, 1993 |
|
Wolfson AH, Brady MF, Rocereto T et al: A gynecologic oncology group randomized phase III trial of whole abdominal Gynecol Oncol. 2007 Nov;107(2):177-85. Epub 2007 Sep 5. |
|
nccn.org |
|
Mansi J, Ramachandra S, Wiltshaw E et al:Case report: Endometrial stromal sarcomas. Gynecol Oncol 36: 113, 1990 |
|
Salazar O, Bonfiglio T, Patten S et al:Uterine sarcomas: Natural history, treatment and prognosis. Cancer 42: 1152, 1978 |
|
Weitmann HD, Knocke TH, Kucera H et al:Radiation therapy in the treatment of endometrial stromal sarcoma. Int J Radiat Oncol Biol Phys 49: 739, 2001 |
|
Ferrer F, Sabater S, Farrus B et al:Impact of radiotherapy on local control and survival in uterine sarcomas: A retrospective study from the Grup Oncologic Catala-Occita. Int J Radiat Oncol Biol Phys 44: 47, 1999 |
|
Chauveinc L, Deniaud E, Plancher C et al:Uterine sarcomas: The Curie Institut Experience: Prognosis factors and adjuvant treatments. Gynecol Oncol 72: 232, 1999 |
|
Stokes S, Bedwinek J, Breaux S et al:Treatment of stage I adenocarcinoma of the endometrium by hysterectomy and irradiation: Analysis of complications. Obstet Gynecol 65: 86, 1985 |
|
Creutzberg CL, van Putten WL, Koper PC et al: The morbidity of treatment for patients with Stage I endometrial cancer: resultsfrom a randomized trial. Int J Radiat Oncol Biol Phys. 2001 Dec 1;51(5):1246-55. |
|
Chao CKS, Grigsby PW, Perez CA et al:Brachytherapy related complications for medically inoperable stage I endometrial carcinoma. Int J Radial Oncol Biol Phys 31: 37, 1995 |